NICE guidance recommends adopting the Axonics SNM System® for refractory OAB
The National Institute for Health and Care Excellence (NICE) has produced guidance recommending the Axonics Sacral Neuromodulation (SNM) System for treating refractory overactive bladder in NHS patients. Evidence supports that the Axonics System improves symptoms and quality of life for overactive bladder patients.
The Axonics® System
Providing a guideline recommended third-line therapy1 for patients suffering with overactive bladder, faecal incontinence and urinary retention, the Axonics System offers the following benefits:
- Long-lasting therapy - approved to function for at least 15 years in the body, which may significantly reduce healthcare costs and patient risk by reducing the need for replacement surgeries.2
- Full-Body MRI conditionally safe - provides patients access to 1.5T and 3T full-body MRI scans under certain conditions*
- Miniaturised neurostimulator - may reduce the risk of implant site pain and decrease patient discomfort, compared to larger implants.3
- Simplified solution designed to optimise therapeutic outcomes - an easy to use patient Remote Control and constant current stimulation allow this therapy to reduce patient fuss and physician burden.4
* See our MRI Guidelines for more information www.axonics.com/MRI
Proven and Sustained Clinical Outcomes
In a European clinical study evaluating the safety and efficacy of the Axonics System for the treatment of overactive bladder, patients completed their 2-year follow-up with sustained efficacy and safety.3
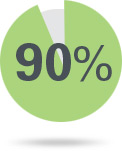
of test responders continued to respond to therapy at 2 years3

Patients averaged a 29-point improvement in quality of life, 3x the bar for clinically meaningful improvement3

No serious device-related adverse events3

Interested in leaning more about NICE's guidance?
The Axonics System is the Product Focus article in the current issue of BJN British Journal of Nursing.
References:
1. AUA/SUFU Guideline: Published Non-Neurogenic OAB Guideline 2019. ASCRS Guidelines on Treatment of FI 20152. Noblett KL, Dmonchowski RR, Vasavada SP, et al. Cost profiles and budget impact of rechargeable versus non-rechargeable sacral neuromodulation devices in the treatment of overactive bladder syndrome. Neurourol Urodyn. 2016;1-7.
2. Noblett KL, Dmonchowski RR, Vasavada SP, et al. Cost profiles and budget impact of rechargeable versus non-rechargeable sacral neuromodulation devices in the treatment of overactive bladder syndrome. Neurourol Urodyn. 2016;1-7.
3. Blok B. et al., Two-year safety and efficacy outcomes for the treatment of overactive bladder using a long-lived rechargeable sacral neuromodulation system. Neurourol Urodyn. 2020;1-7. https://doi.org/10.1002/nau.24317
4. Lettieri C, et al. Clinical outcome of deep brain stimulation for dystonia: constant-current or constant-voltage stimulation? A non-randomized study. Eur J Neurol. 2015 Jun;22(6):919-26
www.Axonics.com
Important Safety Information:
Indications: Axonics SNM Therapy for urinary control is indicated for the treatment of urinary retention and the symptoms of overactive bladder, including urinary urge incontinence and significant symptoms of urgency-frequency alone or in combination, in patients who have failed or could not tolerate more conservative treatments. Axonics SNM Therapy for bowel control is indicated for the treatment of chronic fecal incontinence in patients who have failed or are not candidates for more conservative treatments.
Contraindications: The Axonics SNM System is contraindicated for patients who have not demonstrated an appropriate response to test stimulation; or Patients who are unable to operate the Axonics SNM System.
Warnings: Axonics SNM Therapy is not intended for patients with urinary mechanical obstructions. Diathermy cannot be performed on patients implanted with the Axonics SNM System. The Axonics SNM System is a MRI Conditional system. The following procedures may adversely affect the patient or the Axonics SNM System including: Lithotripsy, Monopolar electro surgery, Microwave and Radiofrequency (RF) ablation, Radiation therapy over the Neurostimulator, and Ultrasound or scanning equipment. Electromagnetic interference can interfere with the function of the Axonics SNM System. Walkthrough metal detectors, security archways, hand-held security wands should not affect the stimulator. Full-body security scanners are considered safe in patients that have the stimulator. Patients should minimize their exposure by not lingering in the immediate area of security systems. The Neurostimulator, Remote Control and Charger contain batteries with chemicals that can cause bodily harm, including severe burns, if exposed to your body. Do not rupture or pierce the devices or use the device that appears damaged or has visible internal components. If swelling or redness occurs near the Charger attachment site, discontinue to the use of Charger and consult your doctor before using the Charger again.
Precautions: The safety and effectiveness of the therapy has not been established for use in pregnant women, the unborn fetus, and during delivery, for pediatric patients (under the age of 18 years for FI and under the age of 16 years for OAB and UR), for patients with neurological disease origins, such as multiple sclerosis or diabetes, or for bilateral stimulation.
Caution: Federal law (USA) restricts this device to sale by, or on the order of, a physician.
© 2020 Axonics Modulation Technologies, Inc. All rights reserved.
26 Technology Drive. Irvine, CA 92618
Toll-Free: 1-877-9-AXONICS
Tel: +1-949-396-6320