Axonics® Privacy Policy
Axonics, Inc. has created this Privacy Policy to document and demonstrate our firm commitment to privacy. This Privacy Policy describes the ways in which Axonics and its affiliates, service providers, and subsidiaries (collectively, “Axonics,” “we,” “our,” or “us”) may collect, use, and disclose personal information obtained or gathered through the use of Axonics websites or services that link to this policy (the “Services”). By using the Services and/or disclosing personal information, you consent to the processing of your personal information as set forth in this Privacy Policy, which is incorporated into our Terms of Use. This Privacy Statement is intended for website users in the United States. We will not sell the personal information that you provide to us.
What Information Do We Collect?
There are various places on the Axonics website where you can elect or sign-up to receive information from Axonics. We may collect the following categories of personal information about you in connection with your use of the Services:
Information You Provide: We and our Service providers collect personal information that you may voluntarily share when you use the Services, including, but not limited to, information about your:
- Contact information, such as your first name, last name, email address, address, phone number;
- Demographic information, including your age, birth date, and gender;
- Professional information, including your employment status, employer information, title, occupation, and professional affiliations;
- Account information, including your username and password;
- Health information, including your health status, diagnoses, treatments, and symptoms;
Information Automatically Collected From You: We and our service providers may automatically collect certain information about you and your online activities when you use the Services. We may collect personal and non-personal information, including, but not limited to, your Internet Protocol address, general geographic location, browser type, operating system, the pages you view on the Services, the pages you view immediately before and after you access the Services, links you follow on our website, and the search terms you enter on the Services.
How Do We Use Your Information?
We may use the information we collect for a number of purposes, including:
- to provide you with products, services, or information you request;
- to provide you with information about the Services or required notices;
- to deliver marketing communications, promotional materials, or advertisements that may be of interest to you;
- to customize your experience when using the Services, such as by providing interactive or personalized elements on the Services and providing you with content based on your interests;
- to improve the Services, such as by better tailoring our content to our users’ needs and preferences;
- to generate and analyze statistics about your use of the Services;
- to answer your questions about the Services, or otherwise communicate with you about the Services;
- for our business purposes, such as monitoring and prevention of fraud, intellectual property infringement, violations of our Terms of Use, violations of law, or other potential misuse of the Services.
When and to Whom Do We Disclose Your Information?
The information that we collect from and about you may be used internally by Axonics and may also be disclosed:
- to third parties that provide services to us in connection with our business operations and that have agreed to keep the information confidential;
- to business partners who offer products or services jointly with us or with our subsidiaries or affiliates;
- as required by law, such as to comply with a subpoena or other legal process, or to comply with government reporting obligations;
- when we believe in good faith that disclosure is necessary (a) to protect our rights, the integrity of the Services, or your safety or the safety of others, or (b) to detect, prevent, or respond to fraud, intellectual property infringement, violations of our Terms of Use, violations of law, or other misuse of the Services; and
- to service providers, advisors, potential transactional partners, or other third parties in connection with the consideration, negotiation, or completion of a corporate transaction in which we are acquired by or merged with another company or we sell, liquidate, or transfer all or a portion of our assets.
Use of Cookies
To enable us to provide customized and personalized services, we and our service providers use cookies and similar tools to store and sometimes track information about you. A cookie is a small amount of data that is sent to your browser from a Web server and stored on your computer. If you do not want the Services to collect information through the use of cookies, you can set your web browser to reject cookies. Each browser is different, so you should check your browser’s “Help” menu to learn how to change your cookie preferences. If you reject or block cookies from the Services, however, the Services may not function as intended.
Use of Google Analytics
Google Analytics uses cookies to track your interactions with our website and online Services. Google then collects that information and reports it back to us. For more information on Google Analytics, visit “HOW GOOGLE USES INFORMATION FROM SITES OR APPS THAT USE OUR SERVICES” located at: https://www.google.com/policies/privacy/partners/.
Do Not Track Signals
California law requires that we share how we respond to website browser “do not track” signals. We do not currently recognize or honor “do not track” signals or other mechanisms that provide a method to opt out of the collection of information across websites or other online services. If we do so in the future, we will describe how we do so in this Privacy Policy. Visit the following website, www.allaboutdnt.org, for more information.
Your California Privacy Rights
If you reside in California and have provided your personally identifiable information to us, you may request information once per calendar year about our disclosures of certain categories of your personally identifiable information to third parties for their direct marketing purposes. Such requests must be submitted to us in writing at
This email address is being protected from spambots. You need JavaScript enabled to view it..
Children's Information
The Services are not intended for or directed to individuals under the age of thirteen (13). If a parent or guardian becomes aware that his or her child has directly provided us with personal information, please contact us by using the contact information below.
Changes to This Privacy Policy
We may update this Privacy Policy from time to time. If we update this Privacy Policy, we will notify you by posting a new Privacy Policy to this page. If we make a material change to our Privacy Policy, we will take reasonable steps to notify you, for example by posting a banner on the Services website, prior to putting the changes into effect.
Contact Us
If you have any questions, please contact us at This email address is being protected from spambots. You need JavaScript enabled to view it..
Effective Date: July 8, 2019
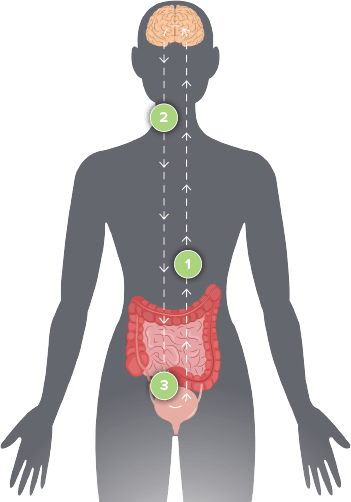
 To determine if Axonics
Therapy is the right treatment option for you, it is important to consult with your doctor.
To determine if Axonics
Therapy is the right treatment option for you, it is important to consult with your doctor. 
 Axonics Therapy starts
with an easy test. The evaluation period allows you to experience the level of symptom relief the therapy may
provide before you commit to a long-lasting therapy.
Axonics Therapy starts
with an easy test. The evaluation period allows you to experience the level of symptom relief the therapy may
provide before you commit to a long-lasting therapy. 
 If you
and your doctor
determine that Axonics Therapy is right for you, you will start your journey towards long-lasting symptom
relief.
If you
and your doctor
determine that Axonics Therapy is right for you, you will start your journey towards long-lasting symptom
relief.