Backed by the pivotal ARTISAN-SNM study and RELAX-OAB study, Axonics® Therapy is clinically proven to help people regain urinary and bowel control.1
ARTISAN-SNM STUDY
The ARTISAN-SNM study is a 129-patient, single-arm, prospective, multicenter, pivotal clinical study approved under an FDA Investigational Device Exemption (IDE) to evaluate the safety and efficacy of Axonics Therapy for urinary dysfunction. The study was conducted in a total of 19 centers, 14 in the United States and 5 in Western Europe.

Click here to view the Journal of Urology article on the 6‑month ARTISAN-SNM study results
VIEW ARTICLE
Click here to view the Neurourology and Urodynamics article on the 2-year ARTISAN-SNM study results
VIEW ARTICLEARTISAN-SNM STUDY 2-Year Results
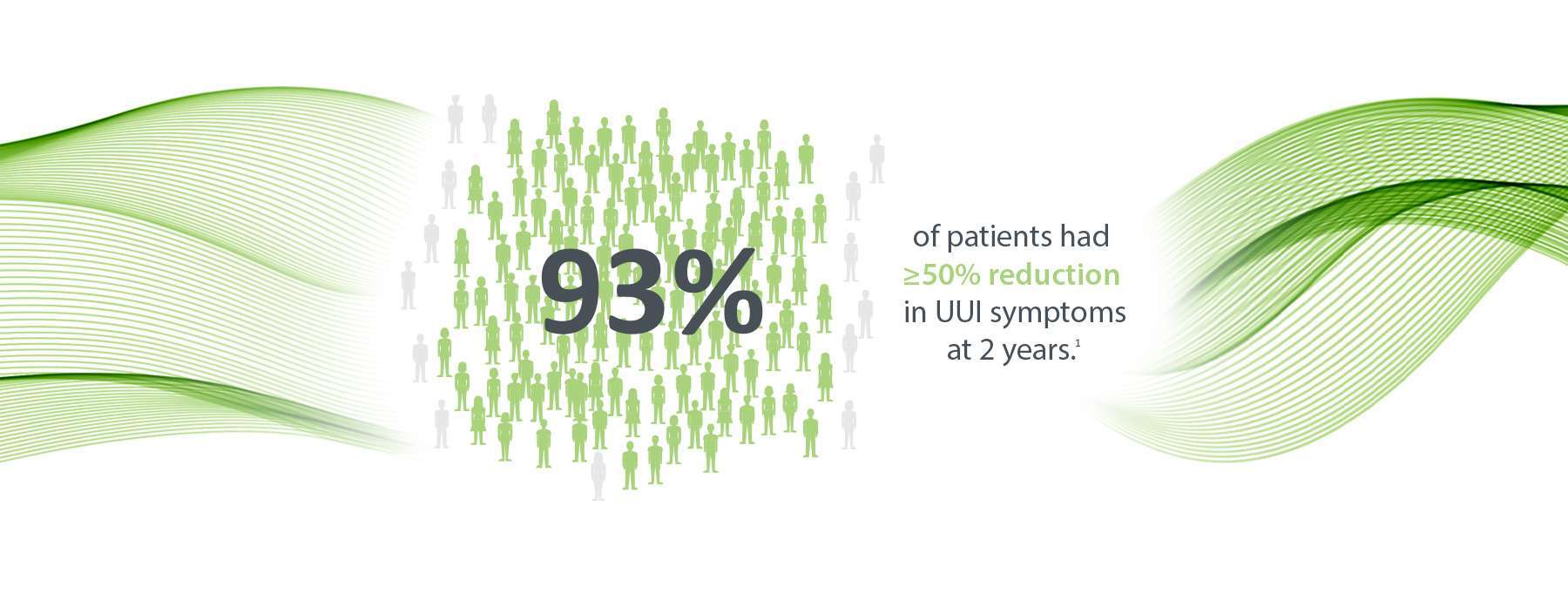
Significant Reductions in UUI Episodes
of implanted patients had ≥50% reduction in UUI symptoms
reduction in UUI episodes across all study patients
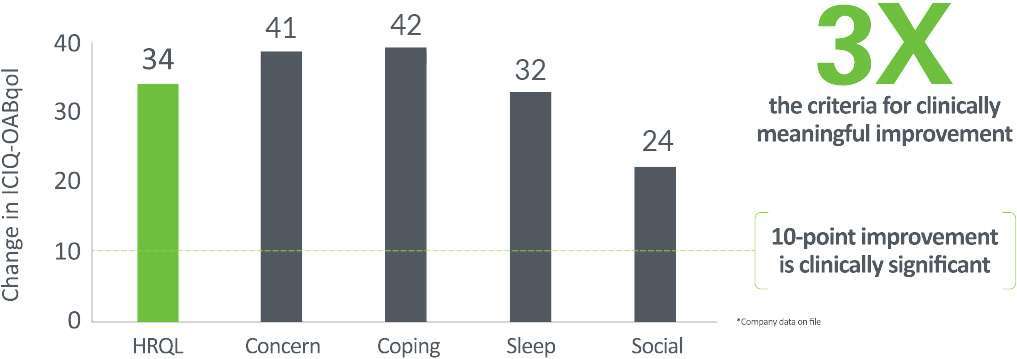
Quality of Life Improvement @ 2 Years
Safety Profile
Treatment with Axonics Therapy was well tolerated, with no serious device-related adverse events reported.
At 2 years:
<1%
rate of lead
migration was
observed
<2%
of patients reported discomfort at the INS site, with no surgical revisions necessary
High Rates of Patient Satisfaction
charging is “EASY”
charging frequency & duration is “ACCEPTABLE”
“SATISFIED” with therapy
would undergo the therapy again with same expected results
RELAX-OAB 2-Year Study Results
The RELAX-OAB study was a 51-patient, post-market clinical follow-up study in Europe designed to test the safety and efficacy of Axonics Therapy for the treatment of overactive bladder. The study was conducted across 7 European centers. Patients in the RELAX-OAB study have completed long-term follow-up out to 2 years, showing sustained efficacy and safety of Axonics Sacral Neuromodulation Therapy.
Proven Durability
of test responders continued to respond to therapy at 2 years
Patients averaged a 29-point improvement in quality of life, 3x the bar for clinically meaningful improvement
No serious device-related adverse events

Click here to download the Neurourology and Urodynamics article on the 2-year RELAX-OAB study results
DOWNLOAD ARTICLEWhat Patients Have to Say About Their Therapy
71 years old
I was hoping to not wear those pads for the rest of my life and not worry when I’m out in public. I can travel now and there’s no concern. That’s what I was hoping for.
45 years old
My frequency is down. My urgency is truly gone.
Results and experiences may vary.
For detailed information regarding our study results, please visit clinicaltrials.gov
ARTISAN-SNM:
NCT03327948
RELAX-OAB: NCT02620410
Additional Literature
- Programming settings and recharge interval in a prospective study of a rechargeable Sacral Neuromodulation system for the treatment of overactive bladder. Blok B, Van Kerrebroeck P, de Wachter S, et al. Neurourol Urodyn. 2018;37:S17–S22. doi:10.1002/nau.23476.
- Three-month clinical results with a rechargeable Sacral Neuromodulation system for the treatment of overactive bladder. Blok B, Van Kerrebroeck P, de Wachter S, et al. Neurourol Urodyn. 2018;37:S9-S16. doi:10.1002/nau.23465.
- The novel Axonics rechargeable Sacral Neuromodulation System: Procedural and technical impressions from an initial North American experience. Elterman DS. Neurourol Urodyn. 2018;37:S1–S8. doi:10.1002/nau.23482.
- A prospective, multicenter study of a novel, miniaturized rechargeable Sacral Neuromodulation system: 12-month results from the RELAX-OAB study. Blok B, Van Kerrebroeck P, de Wachter S, et al. Neurourol Urodyn. 2018;38:689-695. doi:10.1002/nau.23892.
- Cost profiles and budget impact of rechargeable versus non-rechargeable sacral neuromodulation devices in the treatment of overactive bladder syndrome. Noblett KL, Dmochowski RR, Vasavada SP, et al. Neurourol Urodyn. 2016;36:727-733. doi:10.1002/nau.23008.
References:
1. Pezzella A, McCrery R, Lane F, et al. Two-year outcomes of the ARTISAN-SNM study for the treatment of urinary urgency incontinence using the Axonics rechargeable sacral neuromodulation system [published online ahead of print, 2021 Jan 28]. Neurourol Urodyn. 2021;10.1002/nau.24615. doi:10.1002/nau.24615
2. Blok B, Van Kerrebroeck P, de Wachter S, Ruffion A, Van der Aa F, Jairam R, Perrouin-Verbe MA, Elneil S. A prospective, multicenter study of a novel, miniaturized rechargeable sacral neuromodulation system: 12-month results from the RELAX-OAB study. Neurourol Urodyn. 2019 Feb;38(2):689-695.
Questions?
We are here to help answer any questions you may have about Axonics Therapy.